
Graphical representation of the relationship between peptide length and full-length peptide yield. Due to the cyclic nature of the methods of peptide synthesis, the concentration of full-length peptide synthesized in a given reaction is inversely correlated with the length of the proposed peptide. The concentration of full-length peptide synthesized in a reaction is inversely correlated with the length of the proposed peptide therefore, as the length of the peptide increases, the yield is reduced because of the increasing difficulty in purifying the low-abundant product from the crude mixture while peptides 75 amino acids in length can be synthesized, the yield in the synthesis reaction will be poor compared to the yield when synthesizing shorter peptides. With each coupling cycle, a small number of coupling reactions on individual peptides in the reaction mixture fail, resulting in an increasing concentration of truncated peptides (deletions) in the reaction as the length of the peptide being synthesized increases. Additionally, longer peptide sequences require more coupling reactions between the growing peptide and the next amino acid in the sequence. As the length of the peptide increases, so do the amount of impurities that must be removed from the growing peptides after each deprotection-coupling cycle. Although technological advances have enabled current peptide synthesis strategies to be considerably more efficient than ever before, the purity of synthesized crude peptides is limited by the length of the proposed peptide. For example, peptides 10-20 amino acids in length are ideal for antibody preparation, while peptides used for structure/function studies can be more variable.
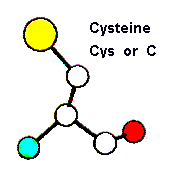
Peptide length is variable and depends on the application for which they are used.


 0 kommentar(er)
0 kommentar(er)
